Structured Authoring Environments and Pharma Industry
 Elmirain Technical Writing on 3/30/2023 — 8 minute read
Elmirain Technical Writing on 3/30/2023 — 8 minute read Elmirain Technical Writing on 3/30/2023 — 8 minute read
Elmirain Technical Writing on 3/30/2023 — 8 minute read

Any technical writer knows that the document creation process can be full of challenges. From using the right terminology to using the appropriate tools, there are a lot of things that can trip you up. For example, technical writing in the pharmaceutical industry is a highly specialized field with very little “creative license” with documents. It is very important for manufacturing documentation, reporting on problems, laboratory test methods, batch production records, etc. to all be crystal clear in their instructions as lives depend on it. This industry is highly regulated, so it is critical to submit accurate and compliant content to regulatory agencies.
However, for many years, the industry has relied on unstructured authoring environments like Microsoft Word and file storage solutions like SharePoint to create and manage content.
Of course, MS Word or SharePoint have served their purpose, but they can also pose significant challenges, such as version control and collaboration issues.
Let’s contemplate the challenges of unstructured environments and how to overcome them and achieve positive results for the pharma industry.
Unstructured authoring environments, such as Microsoft Word, can present a range of challenges for content creators, particularly when working collaboratively or attempting to manage a large volume of content:
To overcome these challenges, organizations may need to consider alternative authoring tools or implement systems and processes that facilitate collaboration and content management.
Structured authoring environments offer several benefits over unstructured authoring environments, particularly when it comes to content creation and management. In the pharmaceutical industry, where accurate and up-to-date documentation is critical for regulatory compliance, structured authoring environments can offer significant advantages:
Structured authoring environments can offer significant benefits for content creation and management in the pharmaceutical industry. By providing greater control over the content, improved version control, and better collaboration features, structured authoring solutions can help organizations to create and manage high-quality documentation more efficiently. With several structured authoring solutions available, pharmaceutical companies have options to choose from to meet their specific requirements.

Implementing structured authoring environments can offer significant benefits to pharmaceutical companies in terms of content creation and management. However, there are several challenges that organizations may face when implementing these solutions. We’ll elaborate upon them below.
To overcome such challenges, there are several strategies that pharmaceutical companies can employ:
Structured documentation solutions have become increasingly popular in recent years, particularly in industries like pharmaceuticals, where compliance with regulatory requirements is critical. The structured authoring is closely connected with the XML format. For example, DITA (Darwin Information Typing Architecture) is an XML-based architecture for authoring, producing, and delivering technical information, designed to provide a standardized way to structure the content for reuse and repurposing. It is not tied to any specific tool or software. The tool-independent content can be created and managed using a variety of tools, as long as those tools support the same standard or markup language. For example, if you create content using DITA architecture, you can use any DITA-compliant authoring tool to create and manage that content. But you must learn DITA for that purpose.
There are special building blocks that are used in the DITA content model. These building blocks include:
The user interface of DITA tools may include a variety of features that help to ensure consistency and standardization across content. For example:
Overall, DITA tools work by providing a structured and standardized approach to content creation and management that enables organizations to create high-quality, consistent, and reusable content. Here are some of the most popular DITA tools:
Despite all the benefits you get from using structured authoring environments, they can be difficult to implement for several reasons:
As you can see, implementing DITA is difficult, time-consuming, and costly. But are there any alternatives when you need to tame the chaos of content management? As it turns out, there are! The fact is, you don’t always need all of the DITA elements – content reuse may be enough to solve 80% of your problems, and then DITA seems like overkill. In such cases, take a look at ClickHelp – this documentation authoring platform solves many documentation management problems more easily than DITA. In particular, it supports single sourcing, content reuse, version control, collaboration features, and the ability to publish content in multiple formats. In addition, this platform already includes hosting – you get not just an editor, but a development and deployment environment! Overall, using tools like ClickHelp can help streamline the content creation and management process, improve collaboration and version control, and ensure regulatory compliance.
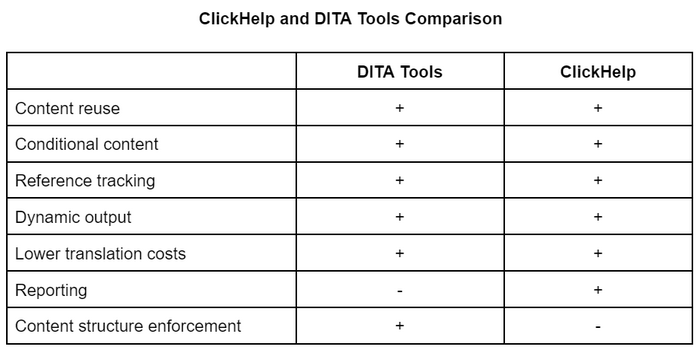
Below is a table that gives you a more explicit comparison of the problems solved by ClickHelp and DITA Tools. As you can see, ClickHelp solves 85% of the problems traditionally thought to require DITA.

Structured authoring environments offer several benefits over unstructured authoring environments, particularly when it comes to content creation and management in the pharmaceutical industry. These benefits include greater control over content, improved version control, and better collaboration features. Strategies for implementing structured authoring environments include involving stakeholders early on, providing training and support, setting realistic goals, identifying quick wins, and ensuring executive buy-in.
Overall, the pharmaceutical industry can greatly benefit from the implementation of structured authoring environments to improve its content creation and management processes, ensure regulatory compliance, and ultimately improve patient outcomes. But structured authoring tools are not the only option here. Take a look at ClickHelp – with support for content reuse and single sourcing, our platform empowers you to solve 85% of your documentation challenges with ease. Plus, with built-in hosting and a comprehensive development environment, ClickHelp is more than just an editor – it’s a complete solution for all your documentation needs. Don’t let the complexity of DITA hold you back – discover the power of ClickHelp today.
Good luck with your technical writing!
ClickHelp Team
Author, host and deliver documentation across platforms and devices
Get monthly digest on technical writing, UX and web design, overviews of useful free resources and much more.
"*" indicates required fields